Background
Patients affected by sickle cell disease (SCD) often present with renal impairment due to the deleterious effects of polymerized red blood cells at the level of the nephron. The resulting glomerular damage leads to proteinuria as well as increased tubular secretion of creatinine, which in turn allows for the use of the urine protein:creatinine ratio (PCR) to monitor disease progression in SCD patients.
Aim
We aimed to evaluate the renal protective effect of various disease-modifying therapies via changes in urine PCR.
Method
This was a retrospective cohort study of patients with SCD (all genotyped, ≥16 years old) at UT Comprehensive Sickle Cell Center during the study period of 2019-2023. Demographics, clinical, and laboratory findings were collected and analyzed. Both the albumin:creatinine ratio (ACR) and PCR are comparable measures of kidney function. We measured PCR at our center due to test availability, and it also allowed the measurement of non-albumin urinary proteins that maybe important for prognosis. PCR was measured once or twice annually. Descriptive statistics are presented in percentages, medians, and interquartile ranges. Changes in urine PCR were calculated based on the difference in initial and recent urine PCR. A positive change means that there was improvement in the most recent urine PCR (i.e., measuring less than the initial urine PCR) or vice versa. An unpaired Student's t test was used to determine the level of significance in differences between initial and recent urine PCR for various disease-modifying drugs, with p <0.05 considered to be statistically significant. Data was analyzed using GraphPad Prism version 9.0.2.
Results
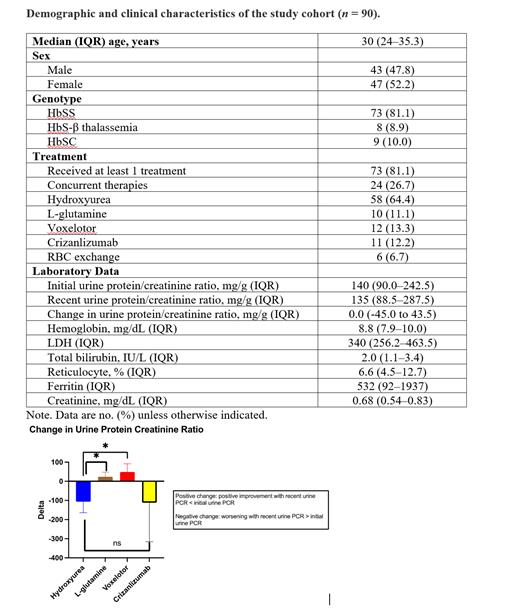
There was a total of 90 study participants eligible for analysis during the study period. Forty-three (47.8%) were male and 47 (52.2%) were female; the median age was 30 (IQR 24-35.3) years. The most common genotype was HbSS (81.1%), followed by HbSC (10%), and HbSB null (8.9%). Patient with sickle cell trait were excluded from this study. The majority of the patients (81.1%) received at least one treatment, and 24 (26.7%) were on combination therapy. Among the various SCD-modifying therapies, hydroxyurea was the most common therapy (64.4%) used, followed by voxelotor (13.3%), crizanlizumab (12.2%), and L-glutamine (11.1%). A detailed analysis is given in Table 1.
The median initial urine PCR was 140 (IQR 90.0-242.5) mg/g. The median recent urine PCR of the study cohort was 135 mg/g (IQR 88.5-287.5), and the median change was 0 (IQR -45.0 to 43.5) mg/g. In stratifying by different disease-modifying drugs, the median change in urine PCR for hydroxyurea was -106.3 mg/g, L-glutamine 5.5 mg/g, voxelotor 19 mg/g, and crizanlizumab -22 mg/g. There was a statistically significant difference in the change in urine PCR for voxelotor and L-glutamine, compared with hydroxyurea (p=0.0361 and 0.0423, respectively) (Figure 1).
Conclusion
This is one of the first retrospective analyses to evaluate the renal protective effect of disease-modifying drugs at our comprehensive sickle cell center. The SCD-modifying therapies voxelotor and pharmaceutical-brand L-glutamine had the highest renal protective abilities and were associated with significant changes in urine PCR, compared with hydroxyurea. Larger studies are needed to corroborate these findings.
Disclosures
Idowu:Pfizer: Consultancy, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Research Funding; Vertex: Consultancy; Novartis: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Forma Therapeutics: Research Funding; Bluebird Bio: Consultancy; Alexion: Research Funding; Agios Pharmaceuticals, Inc.: Research Funding.